About BioQC
BioQC is a global knowledge platform for scientists, researchers and innovators involved in the development and manufacturing of biopharmaceuticals and biologicals.
We deliver in-depth, expert-driven content and facilitate meaningful discussions around the challenges and innovations in CMC and related areas. From FIH studies to market approval, we support every stage of drug development with expert insights delivered via articles, eBooks, webinars, expert forums, and a thriving user community.
At BioQC, we believe that knowledge sharing accelerates progress — leading to more robust processes, faster development timelines, and safer, high-quality medicines for patients worldwide.
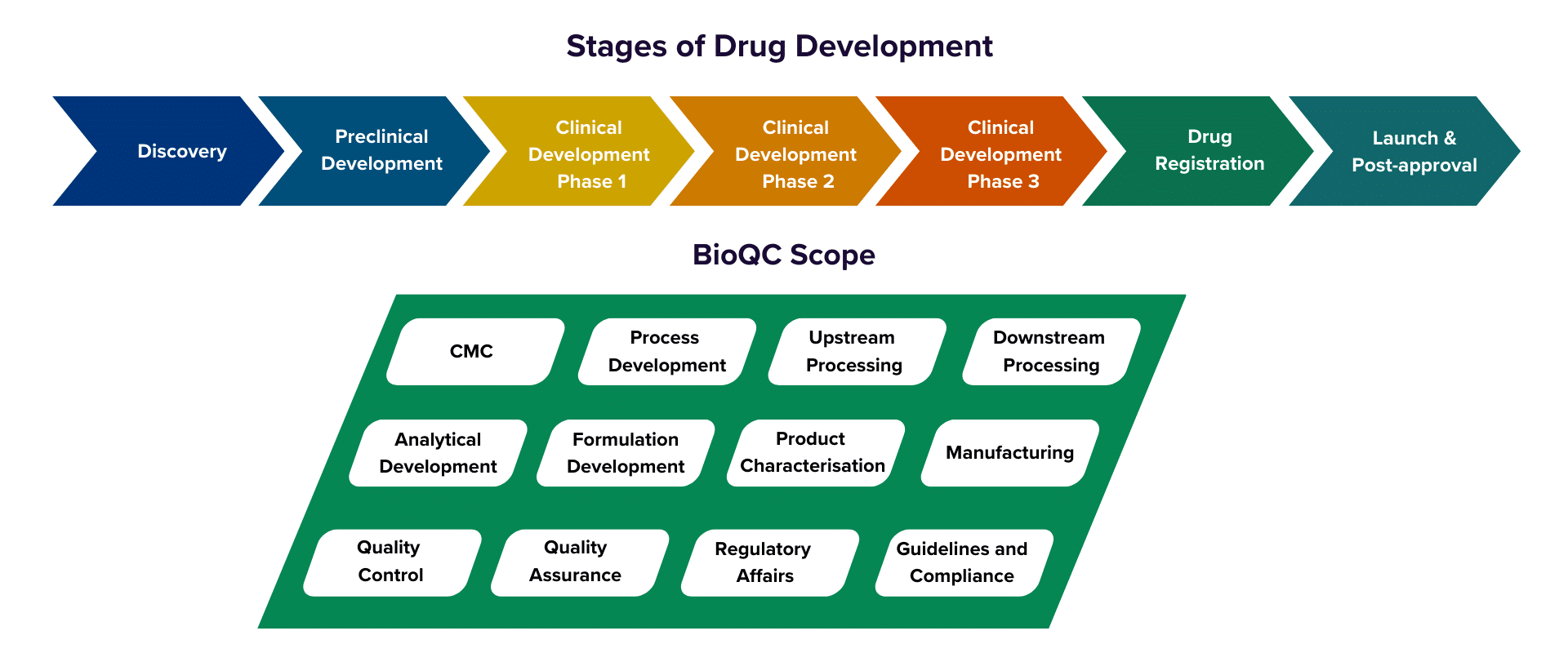
BioQC Scope
We bring together specialists from all key disciplines involved in the lifecycle of biologics, including: Upstream Process Development (USP), Downstream Process Development (DSP), Analytical Development (AD), Product Characterisation, Formulation Development, Quality Control (QC), Quality Assurance (QA), Regulatory Affairs, CMC Management, and Manufacturing.
CMC
- Quality Target Product Profile (QTPP)
- Product specifications
- Patient-centric Pharmaceutical Development
- Personalized Medicine
- Outsourcing
- Biosimilars
- Stability
- Reference standard management
- CMC knowledge management
- Product control strategy
- Critical Quality Attributes (CQAs)
- First in human (FIH)
Process Development
- Protein purification
- Preparative chromatography
- Downstream processing
- Upstream processing
- Scale up
- Process characterisation
- Process Validation
- Process monitoring and controls
- Biosimilars
- PAT (Process Analytical Technology)
- Quality by Design (QbD)
- API / Drug Substance (DS) development
- Drug Product (DP) development
- Starting material characterisation
- Critical process parameters
Downstream Processing
- Protein purification
- Preparative chromatography
- Filtration
- Viral clearance
- Purification
- Process impurities characterisation
Upstream Processing
- Bioreactors
- Cell culture media
- Expression platforms
- Cell culture and fermentation
- Cell-line selection
Biophysical Technologies
- AUC (Analytical Ultracentrifugation)
- DLS (Dynamic Light Scattering)
- AF4-MALS (Asymmetric Flow Field-Flow Fractionation with Multi-Angle Light Scattering)
- FFF (Field-Flow Fractionation)
- SEC (Size Exclusion Chromatography)
- NanoDSF (Differential Scanning Fluorimetry)
- DSC (Differential Scanning Calorimetry)
Analytical Development
- Method development
- Method transfer
- “Method qualification and validation”
- Total error concept
- Method implementation
- Updating Analytical Procedures
- Method Life-Cycle Management (MLCM)
- Lab automation
- ICH Q2(R2) – Validation of Analytical Procedures
- ICH Q14 – Analytical Procedure Development
- Analytical Quality by Design (AQbD)
- Cleaning verification
- Analytical Procedure Control Strategy
- PAT (Process Analytical Technology)
Formulation Development
- Excipients
- Formulation screening
- Extractables and leachables
Manufacturing
- Scale up
- Supply chain
- Facility design and engineering
- Raw material release
- Cleaning verification
- Packaging, labelling and distribution
Analytical Technologies
- HPLC (High Performance Liquid Chromatography)
- RP (Reversed Phase Chromatography)
- HILIC (Hydrophilic Interaction Liquid Chromatography)
- SEC (Size Exclusion Chromatography)
- IEX (Ion Exchange Chromatography)
- Affinity Chromatography
- GPC (Gel Permeation Chromatography)
- CE (Capillary Electrophoresis)
- CZE (Capillary Zone Electrophoresis)
- CGE (Capillary Gel Electrophoresis)
- CE-SDS (Capillary Electrophoresis – Sodium Dodecyl Sulfate)
- LC-MS (Liquid Chromatography – Mass Spectrometry)
- GC-MSxMS (Gas Chromatography – Tandem Mass Spectrometry)
- ICP-MS (Inductively Coupled Plasma – Mass Spectrometry)
- UV-Vis (Ultraviolet-Visible Spectroscopy)
- Raman Spectroscopy
- IR (Infrared Spectroscopy)
- NMR (Nuclear Magnetic Resonance Spectroscopy)
Quality Control
- Method implementation
- Batch testing and release
- Stability testing
- Safety testing
- “Microbiological testing”
- In-process control testing
- Compendial method compliance and testing
- Characterisation support
- “Environmental monitoring”
- “Method qualification, validation and verification”
- Method lifecycle management
- Reference Material Management
- Traceability and data integrity
- Equipment: IQ, OQ, PQ
- Equipment: Maintenance
- Equipment: Updating equipment
- Equipment: Calibration
- Laboratory efficiency (5S)
- Training and competency management
- Out-of-specification (OOS) and out-of-trend (OOT) handling
QA and Regulatory
- Dossier writing
- Regulatory strategies
- Change control/comparability
- Deviations and CAPA’s
- Audits and inspections
- IND/IMPD
- Post-approval changes
Hot Topics
- Biosimilars
- AI
- Analytical Quality by Design (AQbD)
- Digitalisation, Informatics and AI
- Machine learning
Bioanalytical and Cell-based Technologies
- ELISA
- qPCR
- ddPCR (Digital Droplet PCR)
- Gyrolab
- MSD (Meso Scale Discovery)
- Western Blot
- SPR (Surface Plasmon Resonance)
- BLI (Bio-Layer Interferometry)
- TCID50
- Cell-based potency assay
- Flow Cytometry
- Microscopy (light, fluorescence)
- Cell viability assays (MTT, Trypan Blue)
- Mycoplasma detection (PCR or culture)
- Sterility Testing
- Endotoxin Test (LAL or rFC assay)
BioQC Solutions
Through expert forums, webinars, published articles, e-books, a user community, and the eNewsletter, BioQC ensures that cutting-edge insights are easily accessible online to the global scientific community.
This is especially beneficial for scientists, researchers, or industry innovators who cannot attend in-person conferences and events.
BioQC Team

Prof. Dr. Cari Sänger-van de Griend
Scientific Director
Prof. Dr. Cari Sänger–van de Griend is a founder and scientific consultant at Kantisto and an associate professor at Uppsala Universitet in Sweden. Cari holds an MSc in BioPharmaceutical Sciences from Universiteit Leiden and a PhD and Habilitation in Analytical Pharmaceutical Chemistry from Uppsala Universitet. She has previously worked with Astra Pain Control, AstraZeneca, Solvay Pharmaceuticals, and Abbott Healthcare Products, focusing on small molecules, therapeutic proteins, vaccines, and nucleotides. In her daily role, Cari supports industry, institutes, and academia in the realm of (bio)pharmaceutical analytical chemistry and Analytical Quality by Design (AQbD). She is dedicated to sharing knowledge and fostering scientifically sound decisions with the patient in mind.

Dr. Ewoud van Tricht
Scientific Director
Dr. Ewoud van Tricht has over 18 years of experience in the (bio)pharmaceutical industry. He has worked on small molecules, antibodies, proteins, viruses, and cell therapies at companies such as Abbott Healthcare Products, Janssen Vaccines, and Sanofi Cell Therapy. Alongside his full-time career, he completed a Bachelor’s, Master’s, and PhD in Analytical Chemistry. Ewoud specialises in Analytical Quality by Design (AQbD), having developed and implemented strategies to enhance pharmaceutical methods. He is passionate about optimising processes, coaching teams, and driving innovation through AQbD, Agile, and Lean methodologies, always striving for efficient and impactful results.

Dean Graimes
Managing Director
Dean’s career has been entirely focused on media and publishing. His experience encompasses aviation, economics, medical, and scientific fields. He has worked for Reed Elsevier, Thomson Financial, Advanstar, and, most recently, Separation Science, which he co-founded. Dean has developed over 300 online events and forums in pharmaceutical methods and analysis, environmental science, food science, and clinical science, attracting 60,000+ scientists. He is passionate about creating essential content, bringing communities together, and aligning vendors with their end customers.

Jeroen Reiniers
Commercial Director
Jeroen Reiniers began his career in strategy and corporate development at Arthur Andersen & Co., McKinsey & Co., and Reed Elsevier, where he primarily focused on the media, information services, and workflow solutions sectors. Following this, he took on sales, marketing, and business development roles at LexisNexis, Elsevier (ScienceDirect and Voyager ILS), and Thomson Reuters Scientific (Web of Science, Thomson Pharma, and Derwent Innovation). Over the last 15 years, Jeroen’s knowledge and skills have driven the growth of Eclipse Business Media (including Separation Science, Chromatography Forum, and Analytical Training Solutions). His primary strengths encompass fostering customer success, driving sales growth, and reshaping marketing efforts.
Become a BioQC Insider
Subscribe to the free BioQC Insights eNewsletter.
Join the conversation (or start a discussion) on the BioQC user community.
Follow BioQC on LinkedIn.
Advertisement

























