ICH Q14: Guideline Overview for Analytical Procedure Lifecycle Management

The ICH Q14 guideline, formally part of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), represents a step forward in ensuring that analytical methods for pharmaceuticals are scientifically robust, reliable, and adaptable over time. Rooted in ICH Q9 (quality risk management) principles and ICH Q10 (pharmaceutical quality systems), ICH Q14 introduces an enhanced approach that prioritises science-based understanding and continuous improvement. Below is a comprehensive yet accessible overview of its key elements.
What is Analytical Procedure Lifecycle Management?
Analytical procedure lifecycle management refers to the continuum of activities involved in designing, validating, transferring, executing, monitoring, and, when necessary, modifying analytical procedures. Traditionally, these stages were treated as distinct and static, often leading to inefficiencies, redundancies, and risks of failure when unexpected variability arose. The ICH Q14 guideline integrates these stages into a cohesive lifecycle framework, ensuring robust methods capable of consistently delivering accurate, precise results.
This paradigm shift aligns with the broader lifecycle management concepts introduced in other ICH guidelines, such as ICH Q8 (Pharmaceutical Development) and ICH Q12 (Product Lifecycle Management). It emphasises that methods must meet initial validation criteria and remain fit for purpose as circumstances evolve.
Two Approaches in Analytical Procedure Lifecycle Management
The guideline introduces two primary approaches to analytical procedure development: the Minimal Approach and the Enhanced Approach. These approaches provide flexibility, allowing companies to choose a strategy based on the complexity of the analytical procedure, regulatory requirements, and the product’s risk profile.
Minimal Approach
The Minimal Approach focuses on basic validation and method implementation. It generally involves meeting predefined, often generic, acceptance criteria for method performance, typically derived from pharmacopeial requirements. This approach is more straightforward and is suitable for simpler analytical methods that do not involve complex risk factors or substantial variability.
Key Features of the Minimal Approach:
- Use of standard, well-established methods and tools.
- Less emphasis on understanding the underlying variability and interactions in the procedure.
- Focus on compliance with regulatory requirements and acceptance criteria.
- Suitable for routine, high-volume tests where variability is minimal or already well understood.
While this approach is efficient for straightforward methods, it often lacks the flexibility and robustness needed for more complex analytical processes.
Enhanced Approach
The Enhanced Approach, by contrast, provides a much more thorough understanding of an analytical procedure’s performance through Risk Assessment and Design of Experiments (DoE). This approach is driven by a more in-depth scientific knowledge of the process. It uses tools to identify and control critical parameters that influence the outcome of the analytical procedure. The enhanced approach is particularly valuable when there is significant variability in the process or when analytical methods are complex, such as those involved in biologics or advanced drug delivery systems.
As shown in Figure 1, the Enhanced Approach for analytical procedure development systematically evaluates key variables to optimise method performance throughout its lifecycle.
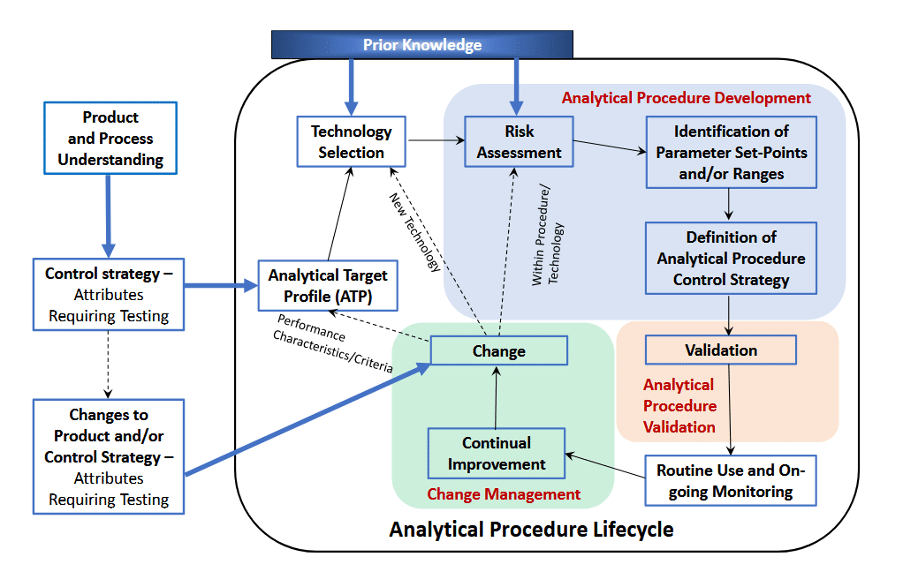 Figure 1: The Analytical Procedure Lifecycle from ICH Q14
Figure 1: The Analytical Procedure Lifecycle from ICH Q14
Key Features of the ICH Q14 Enhanced Approach
(1) Analytical Target Profile (ATP)
The Analytical Target Profile (ATP) is the cornerstone of the enhanced approach. Analogous to the Quality Target Product Profile (QTPP) in product development, the ATP defines the desired performance criteria for an analytical procedure. This includes attributes such as accuracy, precision, sensitivity, and specificity based on the quality attributes of the drug product.
Purpose of ATP:
- Guides the selection and development of analytical methods.
- Serves as a benchmark for validation, verification, and future improvements.
- Supports risk-based decision-making during the lifecycle.
(2) Risk-Based Development
ICH Q14 emphasises using risk assessment tools to identify and prioritise critical method parameters. The approach reduces uncertainty and improves reliability by focusing efforts on parameters that significantly impact the reportable result.
(3) Method Operable Design Region (MODR)
The MODR is the analytical equivalent of a design space, outlining the operational parameters within which the procedure reliably performs. Establishing the MODR involves systematic experimentation to understand the effects of variables (e.g., temperature, pH, and flow rates) on method performance. Although using a MODR is not obligatory, it can have certain advantages.
Benefits of MODR:
- Flexibility in making changes within the MODR without regulatory resubmissions.
- Enhanced method robustness and fewer out-of-specification results.
(4) Continual Improvement
Unlike traditional methods, which often remain static post-validation, the enhanced approach views methods as dynamic. Regular monitoring and data analysis enables ongoing refinement, ensuring methods adapt to new challenges, such as tighter regulatory specifications or changes in manufacturing processes.
Why Choose the Enhanced Approach Over the Minimal Approach?
- Greater Robustness: The enhanced approach minimises the likelihood of failures and out-of-specification results by thoroughly understanding and controlling variability.
- Regulatory Efficiency: By defining MODRs and linking them to the ATP, many post-approval changes can be managed internally, reducing regulatory submissions.
- Lifecycle Adaptability: Methods remain fit-for-purpose even as regulatory requirements or manufacturing conditions change.
| Aspect | Minimal Approach | Enhanced Approach |
|---|---|---|
| Focus | Meeting predefined validation criteria | Lifecycle performance and adaptability |
| Development | Iterative and univariate | Systematic, multivariate, and risk-based |
| Validation | One-time activity | Part of continuous monitoring |
| Flexibility | Limited; changes often require re-validation | Changes within MODR are streamlined |
| Robustness | Reactive to variability | Proactively designed to minimize variability |
Challenges and Opportunities
Challenges:
- Initial Effort: Implementing the enhanced approach requires significant upfront investment in resources, expertise, and experimental design.
- Knowledge Gaps: Many organisations lack sufficient understanding of advanced risk-based and multivariate techniques.
- Regulatory Variability: Global alignment on lifecycle management practices is still evolving, complicating implementation for multi-national companies.
Opportunities:
- Technology Integration: Innovations such as Process Analytical Technology (PAT) and machine learning can further enhance method understanding.
- Harmonization: ICH Q14 promotes consistency in global regulatory expectations, paving the way for streamlined processes.
- Broader Applications: While primarily aimed at pharmaceuticals, these principles are adaptable to biologics, biosimilars, and even non-pharmaceutical industries.
Future Directions
The adoption of ICH Q14, alongside updates to ICH Q2(R2), signals a growing regulatory focus on analytical innovation and lifecycle management. Future developments may include:
- Broader adoption of the ATP and MODR concepts.
- Enhanced integration of digital tools for real-time monitoring and predictive analytics.
- Greater alignment between global regulators to facilitate streamlined implementation.
Conclusion
The ICH Q14 guideline represents a transformative approach to analytical procedure lifecycle management. By focusing on science-based understanding, risk management, and continual improvement, it ensures that analytical methods not only meet current needs but are prepared for future challenges. For industries and regulators alike, adopting these principles will enhance efficiency, consistency, and ultimately, patient safety.
Related Content
Advertisement







